Dec. 12. 2019
Osaka, Japan – December 12, 2019 – Sawai Pharmaceutical Co., Ltd. (Sawai, Head office: Osaka, Japan, President: Mitsuo Sawai) today announced the launch of two generic drugs with five strengths. They will be launched sequentially beginning tomorrow. Sawai’s product line now includes 308 compounds with 746 strengths. Both compounds, “Aprepitant” and “Micafungin Sodium” will be launched for the first time as a generic.
The list of new products
1. Aprepitant Capsules 125 mg [SAWAI] , 80 mg [SAWAI] and Capsule Set [SAWAI]
  |
Generic name | Aprepitant |
|---|---|---|
| Indications | Digestive symptoms (nausea, vomiting) resulting from the administration of antineoplastic agents (cisplatin, etc.), including in the delayed phase | |
| Brand products | EMEND® Capsules 80 mg, 125 mg and Capsule Set | |
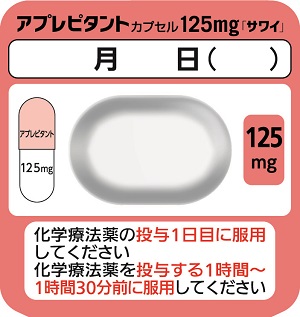
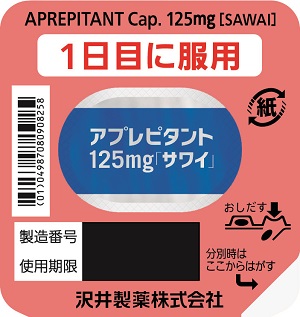
| Striking features | This product is used to suppress digestive symptoms such as nausea and vomiting associated with the administration of anticancer drugs. The capsule size was determined considering the possibility of taking this product in a nauseous state (Capsule 125 mg: total length 17.8 mm, Capsule 80 mg: total length 15.8 mm). The packaging has a patient-friendly design, includes a description of when to take the product and an area to write the date of administration. |
2. Micafungin Na 50 mg [SAWAI] for Infusion and 75 mg [SAWAI] for Infusion
  |
Generic name | Micafungin Sodium |
|---|---|---|
| Indications | Infections caused by Aspergillus sp. and Candida sp. such as fungemia, respiratory mycosis and gastrointestinal mycosis | |
| Brand products | Funguard® 50 mg and 75 mg for Infusion | |
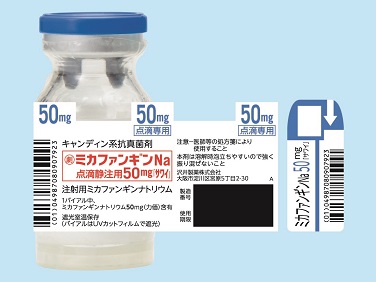
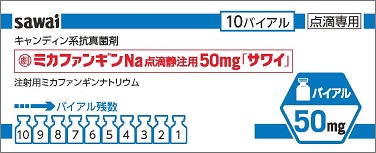
| Striking features | The product is covered with a Sawai-original protector and light-shielding shrink wrapping, both of which protect the product from being exposed to light. These features also protect the vial from breaking, if dropped. The label can be partially separated, and used to write and keep track of the actual dose administered. In addition, the individual box is designed so that the number of remaining vials can be easily checked. |
The products announced in this press release are not approved by the Food & Drug Administration for sale and distribution in the United States.