Osaka, Japan – June 13, 2019 – Sawai Pharmaceutical Co., Ltd. (Sawai, Head office: Osaka, Japan, President: Mitsuo Sawai) today announced the launch of three compounds with seven strengths of generic drugs. They will be launched sequentially beginning tomorrow. Sawai’s product line now includes 306* compounds with 741 strengths. Among the three compounds, one compound, “Blonanserin” will be launched for the first time as a generic.
The list of new products:
1. Silodosin OD Tablets 2 mg [SAWAI] and 4 mg [SAWAI]
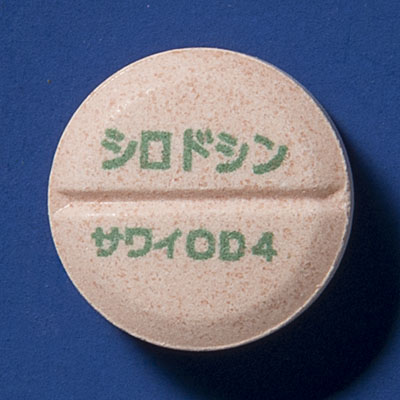 |
Generic name: | Silodosin |
|---|---|---|
| Indications: | Bladder outlet obstruction associated with prostatic hyperplasia | |
| Brand products: | URIEF® OD Tablets 2 mg and 4 mg, and URIEF® Tablets 2 mg and 4 mg | |
| Striking features: | This product is used for bladder outlet obstruction associated with prostatic hyperplasia. These patients may need to limit fluid intake. OD tablets can be taken with or without even with a small amount of water. Sawai aimed to reduce the burden on patients. |
2. Gefitinib Tablets 250 mg [SAWAI]
  |
Generic name: | Gefitinib |
|---|---|---|
| Indications: | Inoperable or recurrent non-small cell lung cancer with mutated EGFR | |
| Brand products: | IRESSA® Tablets 250 | |
| Striking features: | This product adopts non-flap packaging that allows for easy access and storage. We created a packaging design that contributes to the convenience of medical experts. The design liminates the inside flap, features a large opening and a lid that can be easily closed. In addition, generic drugs play an important role in supporting treatment economically, as anti-cancer drug treatment is long-term for patients. Sawai will continue to contribute to all patients battling illness. |
3. Blonanserin Tablets 2 mg [SAWAI], 4 mg [SAWAI], 8 mg [SAWAI] and Blonanserin Powder 2% [AMEL]
 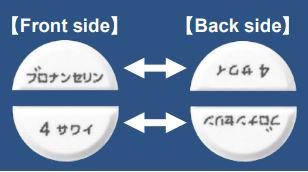 |
Generic name: | Blonanserin |
|---|---|---|
| Indications: | Schizophrenia | |
| Brand products: | Lonasen® Tablets 2mg, 4 mg and 8 mg, and Lonasen® Powder 2% | |
| Striking features: | This product is used for Schizophrenia, and it is necessary to adjust the dosage appropriately depending on the patient’s needs. “Generic name” and “Strength” are printed alternately on the front and back of the divided halves of Blonanserin Tablets 4 mg [SAWAI]. This feature makes it easy to identify and inspect even after being divided on the score. |
A special feature of packages:
Opened design

This inside flap of the box is printed “Opened”.
Its design can signify that it has been opened by bending out and fixing the flap inside the box.
This feature applies to “Silodosin OD Tablets 2 mg [SAWAI] and 4 mg [SAWAI]” and “Blonanserin Tablets 2 mg [SAWAI], 4 mg [SAWAI] and 8 mg [SAWAI]”.
The products announced in this press release are not approved by the Food & Drug Administration for sale and distribution in the United States.